Reproductive Traits and Their Heratabilities in Beef Cattle

Genetic Parameters for Maternal Performance Traits in Commercially Farmed New Zealand Beef Cattle
1
School of Agriculture and Environment, Massey Academy, Private Bag 11222, Palmerston North 4442, New Zealand
2
AbacusBio Ltd., P.O. Box 5585, Dunedin 9058, New Zealand
*
Author to whom correspondence should be addressed.
†
Current address: Beef + Lamb New Zealand Genetics, P.O. Box 5501, Dunedin 9054, New Zealand.
Academic Editor: Michael E. Davis
Received: 22 July 2021 / Revised: 21 August 2021 / Accepted: 24 August 2021 / Published: 26 Baronial 2021
Simple Summary
Enhancing maternal functioning in a beef cattle enterprise can increase overall profitability. Knowledge of the degree of genetic variation in relevant traits is required to inform breeding decisions in commercial environments. The objective of this enquiry was to examine the inheritance of maternal operation traits and to evaluate the trait complementarity amongst reproduction, live weight, hip height, body condition score and maternal weaning weight in 15-month-sometime heifers, 2-year-old cows and mature cows using data collected on commercial New Zealand loma land farms. Results from this study bespeak that almost no genetic variation exists for pregnancy outcomes in 15-month-old heifers and mature cows nether New Zealand farming weather condition just there is potential to amend reproductive performance in two-year-old cows through genetic selection. Cows with greater genetic potential for rebreeding performance after their commencement calving flavour were more likely to have greater alive weight, hip top and body condition score as heifers simply were unlikely to become larger cows at maturity. Cows with genetics for greater maternal weaning weight were more probable to conduct lower body condition and those animals tended to show greater reproductive performance.
Abstract
Maternal functioning is a major commuter of profitability in cow-dogie beefiness cattle enterprises. The aim of this enquiry was to evaluate the inheritance of maternal functioning traits and examine the intercorrelation among reproduction, live weight, hip peak, body status and maternal contribution to calf weaning weight in 15-calendar month-former heifers, 2-year-old cows and mature cows in New Zealand beef herds. Information were collected on a full of 14,241 cows and their progeny on five commercial New Zealand hill country farms. Heritabilities were low for reproductive traits in heifers and mature cows (0–0.06) but were greater in 2-twelvemonth-old cows (0.12–0.21). Torso condition scores were lowly (0.15–0.26) and live weights (0.42–0.48) and hip heights (0.47–0.65) highly heritable in heifers, two-yr-old cows and mature cows. Results indicate that 2-year-old cows with college genetic potential for rebreeding power may have greater genetic merit for live weight, hip height and torso condition as heifers (rg = 0.19–0.54) simply are unlikely to be larger cows at maturity (r1000 = −0.27–−0.ten). The maternal genetic effect on weaning weight had a heritability of 0.20 and was negatively genetically correlated with trunk condition score in lactating cows (rg = −0.55–−0.40) but positively genetically correlated with rebreeding operation (rg = 0.48).
1. Introduction
Beef cows contribute indirectly to the profitability of a beef enterprise by producing calves that are either retained as herd replacements or finished for beef production. In New Zealand commercial farming operations, the profitability of beef cows is low due to low reproduction rates in terms of number of calves weaned per cow per year and loftier feed costs for raising herd replacements for the next generation. Thus, improving maternal and progeny performance is required to increase profitability of the moo-cow-calf unit of measurement.
Potential antagonisms exist between increasing growth in finishing beefiness cattle systems and correlated response in live weight of mature cows [1]. The live weight of cows is often used as a predictor for feed requirements and represents the combined weight of muscle, fat, bones and internal organs [2] and varies by physiological state, frame size and gut-fill [three]. Heavier mature alive weights are associated with higher energy requirements for maintenance, thus an increase would reflect higher costs for maintaining performance [4,five] and, therefore, may affect the profitability of the beefiness cow herd. In times of feed shortage, where feed requirements cannot be met, cows are required to mobilise body free energy reserves. Body condition scoring is a mensurate of body fat reserves and is independent of the previously described factors influencing cow live weight [3,half dozen,7,eight]. Body condition is associated with reproductive functioning of cows and a decline in energy reserves may adversely bear on traits such as pregnancy rate or inter calving interval [ix,10,11]. Dependent on breed and region, research has shown that an increase in alive weight traits tin, although just to a express extent, adversely bear upon reproduction traits including days to calving (ryard = 0.07–0.08) [12,13] or pregnancy rate at 15 months of age (rg = −0.32) [14] whereas other studies reported no impact on reproduction [15].
Selection for improved maternal performance is often inefficient as it relies on traits merely measured on those females retained in the breeding herd and often expressed later on in life [xvi]. Furthermore, most reproductive traits tend to be of low heritability [sixteen,17,18], resulting in reduced prediction accuracy and, thus, constraining the charge per unit of genetic gain. In commercial cattle environments, breeders are oft concerned with the rebreeding power of herd replacements subsequently the first successful calving [xix,xx]. During that time, cows require energy levels to exceed maintenance requirements to achieve growth and milk product in combination with environmental challenges [21]. Without proper management, cows may in some years feel reduced conception rates in their second mating season [20].
Reports on the genetic variability of maternal operation in New Zealand beef cattle are relatively thin and the inheritance of maternal performance traits has non notwithstanding been examined in commercial herds across a range of different environments and years. Better cognition of the inheritance and relationships betwixt key maternal performance traits in commercially farmed beef cattle is needed to make informed breeding decisions. Therefore, the objective of this study was to estimate genetic parameters for reproduction, live weight, hip height, body status and maternal weaning weight traits measured in 15-month-old heifers, 2-year-old cows and mature cows in New Zealand beefiness herds.
2. Materials and Methods
2.1. Dataset and Animal Management
All measurements and related procedures were canonical by the AgResearch Brute Ethics Committee (Approval numbers: 13358, 13373, 13394, 13693, 14031, 14311, 14588, 14851, 15153).
Data available for this inquiry originated from an ongoing beef progeny examination (BPT) by Beef + Lamb New Zealand Genetics conducted on v large-scale commercial hill country farms in New Zealand and initiated in 2014 [22]. The BPT was designed to compare the performance of cattle across a range of unlike breeds and environments.
Records were bachelor from 2014 until 2021 for a total of fourteen,241 animals and the number of records is farther outlined in Table 1. The data contained records on several traits for performance evaluation and records were obtained for the original population of cows in the project as well as all progeny resulting from each mating, and the respective pedigree was recorded. Calves were identified to the dam and sire by DNA parentage verification. Parentage of calves born prior to 2018 was adamant by genotyping progeny and dams using a 120 SNP chip (Zoetis, Auckland, New Zealand) and sires through either 120, 10K or 50K SNP chips (Neogen, Gatton, Commonwealth of australia) and for calves built-in in 2018 or later was verified through 10K SNP chips for progeny and dams and 50K or above for sires (AgResearch GenomNZ, Dunedin, New Zealand).
All replacement heifers were naturally mated in their start 2 breeding seasons, at approximately 15 and 27 months of historic period. But those cows that calved each year were retained in this study. From their third mating onwards, cows received a i-off bogus insemination (AI) at a synchronised oestrus at the offset of mating followed past multi-sire natural mating for the residual of the convenance flavor. Bull breeds used for AI were Angus, Hereford, Stabilizer, Charolais or Simmental and the foundation cows were Angus or Hereford. Details on the synchrony protocol are presented in Weik et al. [10]. The seasons aligned with all-encompassing spring-calving production systems within the Southern Hemisphere such that the mating season began between November and Jan (dependent on the mating date policy on each individual farm). Pregnancy diagnosis (PD) was conducted approximately 90 days following AI and usually coincided with weaning of the calves. Trans-rectal ultrasound scans were conducted past an experienced commercial operator to confirm pregnancy and estimate fetal age. Cows diagnosed as not significant were culled following weaning of their previous calf. Culling was primarily conducted due to unsuccessful pregnancy but was also practiced because of wellness-related reasons. All cows calved in jump with the calving flavor ranging from September until November beyond all herds. Nascency dates were non recorded but calculated for each calf based on the fetal age estimated at PD and assuming a 282 day gestation length [23] whereas simply nascence years were available for the original population of cows.
Animals were kept on pasture year round with little to no supplementary feed. No data have been collected on pasture availability and feed quality due to cattle grazing extensive hill country pastures.
ii.2. Trait Definitions
The number of observations, range of data measures, ways and standard deviations (SD) for recorded traits are outlined in Table ane.
Maternal traits were recorded for all cows present in the herd on the recording 24-hour interval. Traits were pregnancy rate of 15-month-old heifers (HP), days to conception in fifteen-month-old heifers (DtCH), rebreeding performance in ii-year-old cows (RB), days to formulation in two-year-old cows (DtC2), pregnancy charge per unit of mature cows (PR), live weight of fifteen-month-old heifers (HWT), body status score of xv-month-erstwhile heifers (HBCS), hip acme of 15-calendar month-one-time heifers (HH), live weight of ii-year-old cows (WT2), body condition score of 2-yr-one-time cows (BCS2), hip peak of 2-twelvemonth-old cows (HH2), mature cow live weight (MWT), mature cow live weight adjusted for body condition score (MWTBCS), mature cow live weight adjusted for hip height (MWTHH), body condition score of mature cows (BCS), hip height of mature cows (MHH) and weaning weight of calves (WWT). Except for height records where data recording started in 2017 all other traits were recorded throughout the unabridged project. Recording dates for individual traits differed amidst farms involved in the BPT only were consequent for all cows within farm and yr.
Observations for pregnancy outcomes (HP, RB, PR) were recorded as binary traits and were either 0 or 1 coded to represent unsuccessful and successful results, respectively. The HP relates to the per centum of naturally-mated heifers recorded as pregnant amongst all heifers present at PD conducted between 370 and 454 days of age. Too, RB describes the ability of a cow to successfully rebreed between 745 and 841 days of age and is defined as the percent of all 2-year-quondam cows recorded as pregnant of all 2-year-olds present at PD. The trait PR was the percentage of cows aged 3 years or older present at PD that were diagnosed as pregnant.
The reproductive traits DtCH and DtC2 were defined as the number of days from the first of the mating season to the conception day in 15-calendar month-erstwhile heifers and ii-twelvemonth-old cows, respectively. Both measures were from calculations based on estimated fetal historic period recorded at PD used to ascertain probable conception dates. The appointment the showtime female within a mating contemporary group (CG) conceived was taken to exist the start of the convenance flavor for that CG. Further information on CG consignment is provided in the data editing section. To let for the inclusion of not-meaning cows in the analyses, cows that failed to excogitate were assigned a penalty of 21 days from the last conception date within their CG [13,24].
Alive weights (HWT, WT2 and MWT) were recorded using electronic scales. The traits HWT and WT2 were defined as the alive weight of females prior to their starting time or second mating season, respectively. The trait MWT was divers equally the live weight of a moo-cow from three years of age. Measurements for MWT were recorded at three timepoints throughout the annual production cycle: prior to mating (Nov–Jan), at weaning of the calf (February–April) and prior to calving (July–September) and were included equally repeated measures in the analyses. Similarly, body condition score traits (HBCS, BCS2 and BCS) were recorded at the same timepoints inside the product cycle and females were included according to their age as previously described for live weight records. Data for body status scores were obtained by visual cess based on a 1 to 10 scale (1 = emaciated and 10 = obese, Hickson et al. [viii]). Scoring was conducted by an experienced scorer or by the farmer afterwards grooming and under regular calibration to the trained scorer. Hip height records were obtained once a twelvemonth around calving on a continuous calibration using a tape measure out. Based on the age of the cow, records were either HH, HH2 or MHH, according to the group criteria used for alive weight and body condition score records. Records for MWT, BCS and MHH were adjusted to 5 years of age [25] prior to analysis past plumbing equipment a fixed effects model with age and CG as factors in the model. Further adjustments were applied for MWT to either a constant body condition score of 6 or hip height of 130 cm and they are referred to as MWTBCS and MWTHH, respectively. The procedure used was the same as the iv-step procedure presented by Reverter et al. [26] using linear and quadratic furnishings for the covariate (BCS or MHH), with the modification that adjustments were obtained on an private animate being basis as opposed to a CG hateful to business relationship for within CG variation.
Weaning weight of calves was recorded at weaning, at which time the calves' age varied from 110 to 228 days. Linear adjustments to a constant historic period of 200 days of historic period were applied using the same method previously described for MWT adjustments to a abiding BCS or MHH following the arroyo by Reverter et al. [26]. The ancestry of animals built-in within the BPT was generally traced dorsum just one generation, just progeny from naturally-mated heifers with own full-blooded records were weighed at weaning and matched to their sire and dam to allow estimation of the maternal outcome on calf weaning weight. The directly additive genetic effect of WWT is referred to every bit WWTD and the maternal component of weaning weight equally WWTM. The WWTK describes the maternal contribution of the dam to the 200 day weight of the calf (descriptive of genetic potential for milk production [27,28]).
2.3. Data Editing
Recording errors were removed from the existing dataset prior to analyses and twin births were deleted.
For animals that were 2 years of historic period or younger, the estimated birth engagement as adamant past fetal age scanning was used to derive age at data recording in days from birth. For mature cows, the exact birth date was not known and only the nascency year was recorded. Consequently, the recorded birth year was used to compute age of animals in years for traits that were measured on mature cows (MWT, BCS, MHH and PR). Similarly, nascence years were used to calculate age of dam. Grouping was just applied to historic period-in-years parameters such that each individual age represented a separate historic period grade simply animals older than 12 years of age were grouped together due to a limited number of animals in college age classes.
Definitions for CGs are shown in Table 2. Animals with missing information required to define CG or CGs that contained but i animal were removed from the analyses. For binary traits, CGs that contained only the same values (0 or 1) for all animals were excluded. Possible outliers were excluded from the dataset by removing any ascertainment further than three standard deviations from the CG hateful [29]. The original number of records and the size of the dataset post-obit data editing are shown in Figure 1.
All traits were examined for the presence of heterogeneous variances. Linear regression was used to evaluate the relationship between CG mean and SD and a significant relationship was considered evidence for deviation from homogeneity [xxx,31]. Traits were scaled to homogenize the variances where advisable [29] based on the deviation of each tape from the CG mean to the average of the entire dataset [32].
ii.4. Statistical Analysis
Data editing and pre-adjustments of phenotypes were conducted using R version iii.half-dozen [33]. (Co)variance components were estimated using various animate being models in the ASREML iv.1 software package [34]. Heritability, repeatability and correlations (genetic and phenotypic) were calculated from the estimates with their judge standard errors.
The models used for interpretation of genetic parameters were of the general form:
where
is the vector of pre-adjusted observations;
is an incidence matrix relating the fixed effects in
to the observations in
;
,
,
and
are the incidence matrices relating the random effects
for directly condiment genetic,
for maternal genetic,
for permanent environmental and
for maternal environmental effects to observations in
; and
is the vector of balance effects unique to each ascertainment in
.
Expected values of
and variances for the random furnishings included in the model were assumed to be every bit follows:
where
is the numerator relationship matrix,
are identity matrices with their order equal to the number of observations,
the additive genetic variance,
the maternal genetic variance,
the direct-maternal genetic covariance,
the permanent environmental variance,
the maternal ecology variance and
the balance variance.
Given the corporeality of data and number of traits, a single multivariate assay was not feasible such that a variety of uni- and bivariate animal models were used to permit computation of genetic parameter estimates. Variance components for heritability and repeatability estimates were obtained from univariate beast models including all traits on the observed scale. In a second approach all binary traits were analysed using threshold models with a logit-link function. Heritabilities for threshold traits were estimated on the underlying (logit) scale (
) equally follows:
where
/3 is the residual variance on the underlying scale [34,35].
The convergence criterion was the default value used by ASREML such that convergence was presumed when the log-likelihood changed less than 0.002 times the number of iterations and the change of individual variance parameter estimates was below ane%. Initial analysis of variance components considered bivariate animal models including one maternal trait plus WWT to account for any selection prior to data recording. Even so, these models failed to converge in some cases or estimates converged shut to a boundary of the parameter space. For those models that did converge, variance components varied only slightly from the univariate models, such that farther analyses were conducted using simply unmarried-trait models. Genetic and phenotypic correlations between trait pairs were obtained by estimating (co)variance components from bivariate analyses. Linear brute models were assumed amidst all traits [35]. The inclusion of reproductive traits in bivariate analyses was express to those with heritabilities greater than 0.05 [14].
Stock-still and random effects for each trait are displayed in Table iii. Genetic parameters were analysed on an across-brood basis and a brood percentage and heterosis were fitted for each trait. For those traits that were non pre-adjusted to a standard age, age in days was fitted equally a covariate in the terminal model for traits measured on animals two years of age and younger and age in years was fitted equally a factor for mature cow traits. Ancestors were traced dorsum up to two generations. The
matrix comprised xiv,241 individual animals including 423 sires and 4473 dams. No back pedigree was available for the original population of cows in the projection, or the sires used for mating such that the base generation was causeless to be unrelated.
3. Results
iii.i. Univariate Analyses
Estimates of variance components, heritabilities and repeatabilities evaluated in this study are presented in Table 4 for all traits.
Heritability estimates were low or zilch for the binary traits HP (0.00), RB (0.14) and PR (0.00) on the observed scale. Estimates obtained on the underlying calibration using a logit link function differed from those on the observed scale only marginally. Estimates were slightly higher for HP on the logit scale with 0.06 and similar for RB with 0.12. The judge standard errors obtained on the underlying scale, nonetheless, were larger for both traits (0.08–0.11) than those on the observed calibration. The trait PR was non heritable using either assay method.
Live weight and height traits were moderately to highly heritable for xv-month-old heifers, 2-year-sometime cows and mature cows. By and large, variance components and heritabilities were larger for traits observed in mature cows compared with the same traits measured in fifteen-month-old heifers and 2-year-quondam cows and heritabilities ranged from 0.42 to 0.48 for alive weight and from 0.47 to 0.65 for height traits. Adjusting MWT for BCS increased the heritability from 0.48 to 0.57. Variance components were overall lower for MWTBCS compared to MWT and the largest subtract in variance was observed for the permanent environmental issue. The estimated heritability for MWT was lower post-obit hip height adjustments (0.32). Adjustments reduced the additive genetic variance substantially merely had little effect on the permanent ecology variance. Compared to MWT, the residual variance was greater for MWTHH. Heritability estimates for condition score traits were by and large low. Similar to live weight and pinnacle traits, estimates were greater (0.26) for mature cows compared to those obtained for heifers (0.fifteen) but did not differ from the estimates for 2-year-old females (0.25). Repeatability was high overall for mature alive weight traits and ranged from 0.65 to 0.81. Estimates were also high for MHH (0.75) and moderate for BCS (0.42).
The estimated heritability was slightly greater for WWTM (0.20) than for WWTD (0.14). The permanent environmental effect of the dam on WWT was high (0.51). The genetic correlation for directly and maternal genetic upshot of weaning weight was moderate and negative (ram = −0.53).
3.2. Bivariate Analyses
Estimates of heritability obtained from bivariate analyses were by and large similar to those from univariate analyses (Table 5). However, estimates were slightly higher for WT2 (0.52) and HH2 (0.51), likewise equally for MWT (0.51) and MWTBCS (0.61).
The reproductive traits RB and DtC2 were highly correlated (−0.99). This is besides reflected in the correlations of both reproduction traits with live weight, hip top and trunk condition score traits, such that the correlations with RB had the opposite sign to the correlation with DtC2. The exceptions were with WT2, HH2, BCS, MHH and WWTD, all of which were negatively correlated with both reproduction traits. Genetic correlations, withal, were low for each of those trait combinations. Mostly, fifteen-calendar month-old heifer traits were moderately to highly correlated with RB (0.xix–0.54) and DtC2 (−0.57–−0.23). Genetic correlations tended to subtract with increasing age and correlations changed towards the opposing sign, such that live weight, hip height and body condition traits ranged from −0.17 to −0.05 and −0.11 to 0.04 for 2-year-old cows and from −0.32 to −0.10 and −0.08 to 0.17 for mature cows for RB and DtC2, respectively. A moderate genetic correlation has been observed betwixt WWTThou and RB (0.48) and the correlation with DtC2 was low and negative (−0.19).
Genetic correlations for live weight traits were lower among young animals (0.84) compared with correlations with MWT (0.94–0.96). For top traits, genetic correlations were like among sequent ages (0.94–0.97) and decreased with increasing age difference between traits. Estimates amid condition score records were slightly lower compared with live weight and top records and ranged from 0.55 to 0.87.
Live weight and pinnacle traits were highly genetically correlated among all ages (0.61–0.85). Genetic correlations varied betwixt live weight and status score traits dependent on the historic period. Estimates were moderate among HWT and different condition scores (0.26–0.34). The highest genetic correlations were observed betwixt WT2 and either BCS2 (0.57) or BCS (0.62) and betwixt MWT and BCS2 (0.68) whereas no association was observed betwixt HBCS and WT2. Body condition score traits were more often than not only lowly correlated with hip top measures and genetic correlations ranged from −0.16 to 0.15.
Analyses revealed loftier genetic correlations among WWTD and all female person live weight, hip height and trunk condition score traits among all historic period classes (0.51–1.00). 15-month-old heifer and two-twelvemonth-old moo-cow alive weight, hip top and body status score traits were moderately to highly genetically correlated with WWTM and correlations were positive (0.32–0.74) with the exception of BCS2 (−0.40). Genetic correlations amidst WWTYard and mature cow traits were mostly depression to moderate and negative (−0.55–−0.22) simply was low and positive betwixt WWTM and MHH (0.15).
Phenotypic correlations were lower than genetic correlations amid nigh traits. Phenotypic correlations were overall low among female reproduction and alive weight, hip height and body condition traits in xv-calendar month-onetime heifers and 2-year-former cows (−0.07–0.05). Mature moo-cow traits were lowly to moderately correlated with RB and DtC2 on a phenotypic level. Amongst female live weight, hip height and body status score traits correlations were like to genetic correlations and were generally moderate to high between alive weight and height traits (0.42–0.61). Estimates among body condition score traits and live weight traits ranged from a low correlation between HBCS and MWT (0.09) to a loftier correlation between BCS2 and WT2 (0.57). Similar to genetic correlations, only low phenotypic correlations were observed between trunk condition score and tiptop traits (−0.03–0.xviii). Phenotypic correlations among WWT and other live weight, hip height and body status score traits were generally college for 15-calendar month-old heifers than for 2-yr-old or mature cows and decreased with increasing age difference betwixt animals.
4. Discussion
four.i. Effect of Genetics on Reproduction
It is well documented in the literature that most reproduction traits accept merely depression heritability [24,36,37,38] and the aforementioned tendency was observed in the current analyses. The estimates for HP on the underlying scale are consistent with the pooled results reported in the review by Koots et al. [xviii] of 0.05. McAllister et al. [39] reported slightly higher values of 0.17 in Ruddy Angus cattle using a probit link function. On the observed scale heritabilities for yearling pregnancy rates ranged from 0.04 to 0.12 in New Zealand beefiness cattle [37,40]. The trait DtCH basically describes the same trait using a continuous measure of HP while likewise including non-pregnant females. In agreement with the low heritability estimates for HP, estimates for DtCH were equally low. Under the commercial environments the cattle were managed in every bit role of this study, no additive genetic variation could exist detected for heifer pregnancy outcomes.
Rebreeding power is considered a major challenge for offset-calving heifers [19]. Heifers failing to excogitate after their first successful calving are usually culled following weaning of that calf. Due to high costs for replacement animals and limited render from calf weaning upwards until this point, this is the well-nigh expensive fourth dimension to supervene upon females in the convenance herd. Reports, still, are sparse on the genetic potential for rebreeding success in 2-year-erstwhile beefiness cattle. Breeders are often concerned with a reduced pregnancy rate following the first successful calving [20]. This trend, yet, could non be observed in the current study, such that the percentage of animals that conceived to the second mating was higher (92.0%) compared to HP (88.1%). Although heritability was depression for RB on both the observed (0.14) and the underlying calibration (0.12), the results obtained in this study show that genetic variation exists, such that using sires with higher genetic potential for this trait would likely result in a positive response in female progeny. Estimates published by Morris et al. [37] suggest slightly lower heritabilities for pregnancy outcomes for two-twelvemonth-old cows (0.08) on the observed scale. The loftier correlation between RB and DtC2 (−0.99) indicates that they are substantially measures of the same trait. Heritability for DtC2 was slightly greater (0.21) compared to RB in the current study. The greater heritability of two-twelvemonth-onetime cow traits indicates that breeding for greater RB and DtC2 in combination with acceptable management practices has the potential to result in desirable enhancements of rebreeding ability in young herd replacements in the long term.
In the nowadays written report, PR was non heritable on either scale. This is in understanding with the depression estimate of 0.04 reported by Morris et al. [37] and 0.03 past Burrow [36]. The absenteeism of variance estimates for PR may too exist attributable to beast management. Beef cows analysed for PR were field of study to oestrus synchronisation prior to AI in the current written report which may explicate the lack of whatsoever estimable variance in the trait. Goodling et al. [41] institute that oestrus synchronisation reduced the residual variance for pregnancy rate in dairy cattle simply had no substantial consequence on the estimated heritability in their report.
Given the different outcomes for heifers, 2-yr-quondam females and mature cows observed in this report, the commencement 2 matings should exist evaluated as different traits compared to mature cows.
four.2. Live Weight, Hip Summit and Body Condition Score amid 15-Month-Erstwhile Heifers, 2-Year-Old Cows and Mature Cows
Heritability estimates for alive weight, meridian and body condition score traits were college for mature cows compared to heifers and 2-year-olds and those estimates are by and large in agreement with the literature. The heritability of HWT in the current written report is similar to the guess of 0.43 presented by Costa et al. [42] for The states Angus heifers. Heritability estimates for mature alive weight ranged from 0.29 to 0.threescore dependent on breed, historic period, time of the twelvemonth and modelling approach [18,43,44,45,46,47,48]. Mercadante et al. [15] reported lower heritabilities for heifer hip height (0.44) compared to hip height of cows (0.55) in Nellore cattle. The estimated heritability for MHH in the current study is within the range of values presented by Arango et al. [43] of 0.59–0.72 for cows between three and 8 years of age. The heritability of trunk status score in heifers (0.09) presented by Mercadante et al. [15] was slightly lower than for mature cows (0.20) and this aligns with the range of estimates (0.sixteen–0.21) reported past Arango et al. [43] and Johnston et al. [49] for unlike breeds.
Heritability estimates for height were generally greater than for alive weight across all ages in the electric current report and this agrees with previous reports [43,46,50]. The results, however, are in contrast to estimates reported by Meyer [48] where heritabilities for hip height of cows (0.19–0.33) were mostly in the same range as live weight (0.30–0.33) and were lower than the estimates reported in this written report. In agreement with other studies [43,48], heritability estimates for body status score traits were always lower than for live weight or acme traits.
Adjusting MWT for BCS reduced all variance components slightly. The lower heritabilities for MWT compared to MWTBCS can be primarily explained by the divergence in permanent environmental variance and this agrees with the results reported by Arango et al. [43] who suggested that those differences may be owing to variable cow ecology furnishings on body fat reserves that are better accounted for when adjusting MWT to a constant BCS. The college heritability of MWTBCS compared to MWT agrees with the results reported past Arango et al. [43] of 0.54 and Nephawe et al. [46] of 0.57. Meyer [48] also reported slightly higher estimates following BCS adjustments simply estimates were overall lower (0.31–0.34) than in the current study (0.57).
Estimates on MWT adapted to a constant height are rare in the literature. The results of 0.32 plant in this report hold with the estimates reported by Hickson and Pitchford [51] of 0.25–0.35 in Australian Angus cows. Adjusting MWT to a standard hip summit reduced the heritability considerably and this is primarily due to a reduction in the additive genetic variance. Hip summit is a highly heritable trait and taking out the effect of differences in hip elevation may remove most of the variation in MWT that is explained through skeletal size. The majority of furnishings that influence MWT following hip height adjustments are likely related to muscle and fatty deposition and heritability for those traits tends to be lower. Heritability for MWTHH (0.32) was slightly greater than for BCS (0.26) in the current study. Genetic and phenotypic correlations among MWTHH and other female live weight, hip meridian and body condition score traits, however, indicate that MWTHH behaves in a similar manner to BCS. Hickson and Pitchford [51] found that estimating MWTHH did non add pregnant value to BCS equally a option criterion for improved status. In practice, height may be a more than complex trait to measure compared to BCS. The reward of this method is, however, that measuring pinnacle is a more standardised method and does not require a trained technician to accurately record body free energy reserves, thus would remove bias due to subjective assessment of animals.
Repeatability estimates were high for mature cow live weights (0.65–0.81) and this is within the range of estimates reported in the literature between 0.57 and 0.85 using REML [43,47,48,49]. Couch [36], even so, reported a higher repeatability for moo-cow live weight of 0.93. Repeatability of MHH was similar compared to alive weight traits and this is in agreement with the reported estimates of 0.75 by Arango et al. [43] and 0.73 to 0.77 by Meyer [48]. Amid alive weight, hip elevation and body condition traits, BCS was the least repeatable in the current report (0.42) and the same has been observed in previous research [48]. The estimated repeatability was within the range (0.32–0.52) reported by Johnston et al. [49] for different beef cow breeds.
Genetic correlations were high among live weight and summit traits and this was expected [43,48,50]. In understanding with literature findings [43], the genetic correlations were low betwixt body condition score and top traits. Moderate to high genetic correlations were estimated in the electric current study betwixt live weight and body condition score traits and this agrees with the estimates (0.49–0.65) reported by Johnston et al. [49] across dissimilar breeds. Thus, selecting for reduced mature live weight (without aligning for BCS) to decrease maintenance requirements tin can reduce trunk status score. Previous research has shown that lower conditioned mixed-anile cows may feel reduced reproductive performance compared to better conditioned cows on a phenotypic level [ten]. Too reproduction related reasons, other rationales, such every bit health and animal welfare, exist for breeders to produce cows that are able to maintain or increase BCS. The current study indicates that breeding for limited mature cow live weight and size to reduce maintenance requirements while at the aforementioned fourth dimension maintaining BCS requires an culling approach to using this data to prevent any unfavourable selection against correlated traits with potential bear upon on productivity.
four.3. Clan amidst Reproduction, Alive Weight, Hip Height and Body Condition
Results from the current study suggest a positive genetic correlation between RB and live weight, hip height and body condition score traits in 15-month-old heifers, indicating that cows with a higher genetic potential for RB performance would be likely to prove increased HWT, HBCS and HH. With increasing historic period, the correlation decreased among RB or DtC2 and the respective live weight, hip height and body condition score traits toward the opposing sign. The correlations, however, were only low in ii-year-old cows (−0.17–0.04) and low to moderate in mature cows (−0.32–0.17). Given the big standard errors among those correlations with both reproductive traits, females with genetically superior reproductive performance at rebreeding are unlikely to exhibit an unfavourable reduction in body free energy reserves as a cow. Comparing the genetic correlations of reproductive traits with other heifer and mature cow traits indicates that improvement in RB and DtC2 may result in faster growing, amend conditioned heifers only those heifers are unlikely to become bigger cows. The correlation between DtC2 and MWT was low and positive (0.08) in the current study and was non significantly unlike from zero. Couch [36] also reported a low just negative genetic correlation of −0.fifteen between mature alive weight and days to calving. Co-ordinate to Mercadante et al. [fifteen], pick for growth-related traits would non compromise reproductive performance, which in their case was measured as days to calving and calving success. More often than not, results from the current analyses tend to concord with their assertion. Comparing results of DtC2 analysed in the electric current study with days to calving, yet, needs to be treated with caution equally gestation length will explain part of the variation in days to calving.
4.four. Maternal Contribution to Dogie Weaning Weight and Its Impact on Reproduction, Live Weight, Hip Height and Torso Status Score
Heritability estimates for WWT found in the electric current report agree with the estimates reported past Splan et al. [52] for the straight genetic effect of WWT (0.xiv) and the maternal genetic upshot (0.19) in US crossbred beef cattle. Similar estimates were reported past Burrow [36] in Australian Belmont cattle with 0.17 for the straight genetic and 0.34 for the maternal genetic effect of weaning weight. Those values are consistent with those reported past Morris et al. [37] of 0.fourteen for directly and 0.35 for maternal weaning weight. Meyer et al. [53] reported slightly college estimates for direct weaning weight (0.22) and lower heritabilities for the maternal component of weaning weight (0.18) in Australian Hereford cattle. The permanent ecology outcome of the dam was also lower in their written report (0.20) compared to the result in the current analysis (0.51). Results from this study indicate that a large proportion of differences in calf weaning weights are owing to the genetics and permanent environments of the dam subsequently the effects of CG and historic period of dam have been removed.
The additive maternal genetic correlation between WWTD and WWTOne thousand in the electric current study was moderate and negative (−0.53) and an unfavourable correlation has been previously reported past several researchers [27,36,54,55,56]. Genetic correlations betwixt the direct genetic component of WWTD and live weight, hip meridian and body status score traits are similar to those correlations amid other alive weight, height and body status score traits and this was expected. The highest correlations were estimated between WWTD and live weight and acme traits of all ages which agrees with the literature [27,56,57] and estimates were generally lower among WWTD and body status score traits.
The contribution of the dams to the WWT of their calves in terms of milk product has been previously described in the literature but correlations with other maternal and production blazon traits are sparse. In the current written report, the genetic correlations were moderate to loftier betwixt WWTM and live weight, hip height and trunk condition score traits in 15-months-quondam heifers. Once females calve for the commencement time, cows with genetics for higher WWTYard are likely to exhibit lower body condition scores every bit shown in the current written report. Results from the current study are in agreement with Wolcott et al. [57] who reported a moderate negative genetic correlation between the maternal component of WWT and body condition score (−0.50) as well as depression correlations with weight (0.xv) and elevation traits (0.19) for 2-twelvemonth-old Brahman cows recorded prior to their second mating season. The positive genetic correlations among WWTGrand and any pinnacle mensurate observed in this study indicate that cows with greater genetic merit for height are more likely to exhibit greater overall WWTM and, therefore, are likely to evidence greater milk production. Those correlations were, notwithstanding, low at maturity and this has been previously reported for dairy cows [58]. The negative genetic correlation between MWT and WWTYard in the current study agrees with the estimates reported by Mwansa et al. [27] of −0.17 and Kaps et al. [54] of −0.34.
5. Conclusions
This written report has shown that there is potential to meliorate reproductive performance in two-year-one-time cows through choice. Animals with greater genetic potential for rebreeding performance as 2-year-olds tend to be heavier, taller heifers at 15 months of age with greater body fat reserves but those animals do not tend to be genetically bigger at maturity and this may reduce cow maintenance requirements. Traits measured on heifers prior to their first calving can be substantially different from similar traits measured in cows post-obit their first and subsequent calving. This should exist taken into account when considering maternal performance for genetic evaluations and option program design.
Author Contributions
Conceptualization, F.W., R.E.H., J.A.A., S.T.M. and D.J.G.; methodology, F.Westward., J.A.A., R.East.H., South.T.M. and D.J.Thousand.; software, F.W.; validation, F.Westward. and J.A.A.; formal analysis, F.W., J.A.A., R.E.H. and D.J.Yard.; investigation, J.A.A.; resources, J.A.A. and R.Due east.H.; data curation, F.W.; writing—original draft preparation, F.West.; writing—review and editing, F.W., R.E.H., J.A.A., South.T.M. and D.J.Grand..; visualization, F.W.; supervision, R.E.H., J.A.A., S.T.Chiliad. and D.J.Yard.; project assistants, J.A.A.; funding acquisition, J.A.A. and R.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This inquiry was funded by Beef + Lamb New Zealand Genetics (BPT2014) and the primary author was funded by the Schoolhouse of Agronomics and Surround, Massey University.
Institutional Review Lath Statement
This study was conducted according to the guidelines of the AgResearch Code of Ethical Bear for the Use of Animals for Research, Testing and Educational activity, and canonical by the AgResearch Animal Ethics Committee (Approval numbers: 13358 (canonical on eighteen November 2014), 13373 (approved on eighteen Nov 2014), 13394 (approved on 18 November 2014), 13693 (approved on 17 Nov 2015), 14031 (canonical on 25 October 2016), 14311 (approved on 9 November 2017), 14588 (approved on 13 Nov 2018), 14851 (approved on 28 November 2019), 15153 (approved on nineteen November 2020)).
Data Availability Statement
Third-Political party Data. Restrictions apply to the availability of these information. Data were obtained from Beef + Lamb New Zealand Genetics.
Acknowledgments
The authors gratefully acknowledge the contributions of Craig Foote, Geoff Purchas, Luke Proctor and all farm managers, technical and support staff, who contributed to farm management practices and data drove.
Conflicts of Interest
The authors declare no conflict of interest. The funders approved the blueprint of the written report and assisted with the drove of data but had no role in the analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Archer, J.A.; Arthur, P.F.; Parnell, P.F.; van de Ven, R.J. Upshot of divergent selection for yearling growth charge per unit on female reproductive performance in Angus cattle. Livest. Prod. Sci. 1998, 57, 33–xl. [Google Scholar] [CrossRef]
- Owens, F.N.; Gill, D.R.; Secrist, D.S.; Coleman, S.W. Review of some aspects of growth and evolution of feedlot cattle. J. Anim. Sci. 1995, 73, 3152–3172. [Google Scholar] [CrossRef]
- Morris, S.T.; Kenyon, P.R.; Burnham, D.L. A comparing of two scales of body condition scoring in Hereford × Friesian beef breeding cows. Proc. Northward. Z. Grassl. Assoc. 2002, 64, 121–123. [Google Scholar] [CrossRef]
- Ochsner, K.P.; MacNeil, M.D.; Lewis, R.M.; Spangler, M.Fifty. Economic pick index development for Beefmaster cattle II: General-purpose breeding objective. J. Anim. Sci. 2017, 95, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Smeaton, D.C.; Bown, M.D.; Clayton, J.B. Optimum liveweight, feed intake, reproduction, and dogie output in beef cows on North Island hill country, New Zealand. North. Z. J. Agric. Res. 2000, 43, 71–82. [Google Scholar] [CrossRef]
- Wagner, J.J. Carcass Composition in Mature Hereford Cows: Estimation and Influence on Metabolizable Energy Requirements for Maintenance during Winter. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, U.s.a., 1984. [Google Scholar]
- Bishop, D.K.; Wettemann, R.P.; Spicer, L.J. Body free energy reserves influence the onset of luteal activity afterwards early weaning of beef cows. J. Anim. Sci. 1994, 72, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.East.; Morris, S.T.; Thomson, B.C. Beef Cow Body Condition Scoring; Beef + Lamb New Zealand: Wellington, New Zealand, 2017. [Google Scholar]
- Osoro, K.; Wright, I.A. The consequence of body condition, live weight, breed, age, calf performance, and calving appointment on reproductive performance of spring-calving beef cows. J. Anim. Sci. 1992, 70, 1661–1666. [Google Scholar] [CrossRef]
- Weik, F.; Archer, J.A.; Morris, S.T.; Garrick, D.J.; Hickson, R.E. Human relationship between torso condition score and pregnancy rates following artificial insemination and subsequent natural mating in beefiness cows on commercial farms in New Zealand. Northward. Z. J. Anim. Sci. Prod. 2020, 80, 14–20. [Google Scholar]
- Morris, S.T.; Morel, P.C.H.; Kenyon, P.R. The issue of individual liveweight and status of beef cows on their reproductive performance and nascency and weaning weights of calves. N. Z. Vet. J. 2006, 54, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Forni, South.; Albuquerque, L.M. Estimates of genetic correlations between days to calving and reproductive and weight traits in Nelore cattle. J. Anim. Sci. 2005, 83, 1511–1515. [Google Scholar] [CrossRef]
- Johnston, D.J.; Bunter, G.L. Days to calving in Angus cattle: Genetic and environmental effects, and covariances with other traits. Livest. Prod. Sci. 1996, 45, xiii–22. [Google Scholar] [CrossRef]
- Wolcott, G.L.; Johnston, D.J.; Barwick, S.A. Genetic relationships of female reproduction with growth, body composition, maternal weaning weight and tropical adaptation in two tropical beef genotypes. Anim. Prod. Sci. 2014, 54, 60–73. [Google Scholar] [CrossRef]
- Mercadante, Grand.E.Z.; Packer, I.U.; Razook, A.Thousand.; Cyrillo, J.N.South.M.; Figueiredo, L.A. Directly and correlated responses to selection for yearling weight on reproductive performance of Nelore cows. J. Anim. Sci. 2003, 81, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Cammack, K.G.; Thomas, M.G.; Enns, R.One thousand. Reproductive traits and their heritabilities in beef cattle. Prof. Anim. Sci. 2009, 25, 517–528. [Google Scholar] [CrossRef]
- Johnston, D.J. Genetic improvement of reproduction in beef cattle. In Proceedings of the 10th Earth Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014; pp. 17–22. [Google Scholar]
- Koots, K.R.; Gibson, J.P.; Smith, C.; Wilton, J.W. Analyses of published genetic parameter estimates for beefiness production traits. 1. Heritability. Anim. Brood. Abstr. 1994, 62, 309–338. [Google Scholar]
- Valente, T.S.; Albito, O.D.; Sant'Anna, A.C.; Carvalheiro, R.; Baldi, F.; Albuquerque, L.G.; Paranhos da Costa, M.J.R. Genetic parameter estimates for temperament, heifer rebreeding, and stayability in Nellore cattle. Livest. Sci. 2017, 206, 45–50. [Google Scholar] [CrossRef]
- Hickson, R.Eastward.; Anderson, W.J.; Kenyon, P.R.; Lopez-Villalobos, North.; Morris, Due south.T. A survey of beef cattle farmers in New Zealand, examining management practices of primiparous breeding heifers. N. Z. Vet. J. 2008, 56, 176–183. [Google Scholar] [CrossRef]
- Boligon, A.A.; Ayres, D.R.; Pereira, R.J.; Morotti, Northward.P.; Albuquerque, L.G. Genetic associations of visual scores with subsequent rebreeding and days to commencement calving in Nellore cattle. J. Anim. Breed. Genet. 2012, 129, 448–456. [Google Scholar] [CrossRef]
- Weik, F.; Archer, J.A.; Morris, S.T.; Garrick, D.J.; Miller, S.P.; Boyd, A.One thousand.; Cullen, N.G.; Hickson, R.E. Live weight and body condition score of mixed-aged beef breeding cows on commercial colina land farms in New Zealand. N. Z. J. Agric. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Burris, Yard.J.; Blunn, C.T. Some factors affecting gestation length and birth weight of beefiness cattle. J. Anim. Sci. 1952, xi, 34–41. [Google Scholar] [CrossRef]
- Meyer, Chiliad.; Hammond, Grand.; Parnell, P.F.; MacKinnon, M.J.; Sivarajasingam, S. Estimates of heritability and repeatability for reproductive traits in Australian beef cattle. Livest. Prod. Sci. 1990, 25, 15–30. [Google Scholar] [CrossRef]
- Graser, H.U.; Tier, B.; Johnston, D.J.; Barwick, Southward.A. Genetic evaluation for the beefiness manufacture in Australia. Aust. J. Exp. Agric. 2005, 45, 913–921. [Google Scholar] [CrossRef]
- Reverter, A.; Johnston, D.J.; Graser, H.-U.; Wolcott, G.50.; Upton, West.H. Genetic analyses of live-animal ultrasound and abattoir carcass traits in Australian Angus and Hereford cattle. J. Anim. Sci. 2000, 78, 1786–1795. [Google Scholar] [CrossRef]
- Mwansa, P.B.; Crews Jr, D.H.; Wilton, J.W.; Kemp, R.A. Multiple trait selection for maternal productivity in beef cattle. J. Anim. Brood. Genet. 2002, 119, 391–399. [Google Scholar] [CrossRef]
- Meyer, One thousand.; Carrick, Thousand.J.; Donnelly, B.J.P. Genetic parameters for milk production of Australian beef cows and weaning weight of their calves. J. Anim. Sci. 1994, 72, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Brownish, D.J.; Atkins, K.; Huisman, A.E. Expression of body weight, fleece weight and fibre diameter in beyond flock genetic evaluation. Proc. Assoc. Advmt. Anim. Breed. Genet. 2005, sixteen, 84–87. [Google Scholar]
- Everett, R.West.; Keown, J.F. Mixed model sire evaluation with dairy cattle—Experience and genetic gain. J. Anim. Sci. 1984, 59, 529–541. [Google Scholar] [CrossRef]
- Lopez-Villalobos, N.; Garrick, D.J.; Harris, B.50.; Blair, H.T. Accounting for scale effects in genetic evaluation of dairy cattle. Proc. N. Z. Soc. Anim. Prod. 1994, 54, 275–279. [Google Scholar]
- Pickering, N.K.; Dodds, Thou.G.; Blair, H.T.; Hickson, R.E.; Johnson, P.L.; McEwan, J.C. Genetic parameters for production traits in New Zealand dual-purpose sheep, with an accent on dagginess. J. Anim. Sci. 2012, 90, 1411–1420. [Google Scholar] [CrossRef]
- R Cadre Team. R: A Linguistic communication and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on xx June 2020).
- Gilmour, A.; Gogel, B.; Cullis, B.; Welham, S.; Thompson, R. ASReml User Guide Release 4.one Structural Specification; VSN International Ltd.: Hemel Hempstead, United kingdom, 2015. [Google Scholar]
- Johnston, D.J.; Barwick, S.A.; Fordyce, G.; Holroyd, R.G.; Williams, P.J.; Corbet, N.J.; Grant, T. Genetics of early and lifetime almanac reproductive functioning in cows of 2 tropical beef genotypes in northern Australia. Anim. Prod. Sci. 2014, 54, 1–xv. [Google Scholar] [CrossRef]
- Couch, H.Thou. Variances and covariances betwixt productive and adaptive traits and temperament in a composite breed of tropical beef cattle. Livest. Prod. Sci. 2001, 70, 213–233. [Google Scholar] [CrossRef]
- Morris, C.A.; Wilson, J.A.; Bennett, K.L.; Cullen, N.G.; Hickey, S.M.; Hunter, J.C. Genetic parameters for growth, puberty, and beef cow reproductive traits in a puberty pick experiment. N. Z. J. Agric. Res. 2000, 43, 83–91. [Google Scholar] [CrossRef]
- Cavani, 50.; Garcia, D.A.; Carreño, L.O.D.; Ono, R.K.; Pires, M.P.; Farah, 1000.Chiliad.; Ventura, H.T.; Millen, D.D.; Fonseca, R. Estimates of genetic parameters for reproductive traits in Brahman cattle breed. J. Anim. Sci. 2015, 93, 3287–3291. [Google Scholar] [CrossRef]
- McAllister, C.G.; Speidel, S.Eastward.; Crews Jr, D.H.; Enns, R.M. Genetic parameters for intramuscular fat per centum, marbling score, scrotal circumference, and heifer pregnancy in Carmine Angus cattle. J. Anim. Sci. 2011, 89, 2068–2072. [Google Scholar] [CrossRef]
- Morris, C.A.; Cullen, North.G. A annotation on genetic correlations between pubertal traits of males or females and lifetime pregnancy rate in beef cattle. Livest. Prod. Sci. 1994, 39, 291–297. [Google Scholar] [CrossRef]
- Goodling, R.C.; Shook, G.Due east.; Weigel, Thousand.A.; Zwald, N.R. The upshot of synchronization on genetic parameters of reproductive traits in dairy cattle. J. Dairy Sci. 2005, 88, 2217–2225. [Google Scholar] [CrossRef]
- Costa, R.B.; Misztal, I.; Elzo, One thousand.A.; Bertrand, J.K.; Silva, Fifty.O.C.; Łukaszewicz, Thousand. Estimation of genetic parameters for mature weight in Angus cattle. J. Anim. Sci. 2011, 89, 2680–2686. [Google Scholar] [CrossRef]
- Arango, J.A.; Cundiff, Fifty.V.; Van Vleck, L.D. Genetic parameters for weight, weight adapted for torso condition score, superlative, and body condition score in beef cows. J. Anim. Sci. 2002, 80, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Kaps, M.; Herring, W.O.; Lamberson, West.R. Genetic and environmental parameters for mature weight in Angus cattle. J. Anim. Sci. 1999, 77, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Albuquerque, 50.G.; Mercadante, M.E.Z.; Lobo, R.B. Models for genetic evaluation of Nelore cattle mature body weight. J. Anim. Sci. 2008, 86, 2840–2844. [Google Scholar] [CrossRef] [PubMed]
- Nephawe, One thousand.A.; Cundiff, L.V.; Dikeman, M.E.; Crouse, J.D.; Van Vleck, L.D. Genetic relationships between sex-specific traits in beef cattle: Mature weight, weight adjusted for body condition score, meridian and body condition score of cows, and carcass traits of their steer relatives. J. Anim. Sci. 2004, 82, 647–653. [Google Scholar] [CrossRef]
- Morris, C.A.; Baker, R.L.; Johnson, D.Fifty.; Carter, A.H.; Hunter, J.C. Reciprocal crossbreeding of Angus and Hereford cattle iii. Cow weight, reproduction, maternal operation, and lifetime production. N. Z. J. Agric. Res. 1987, thirty, 453–467. [Google Scholar] [CrossRef]
- Meyer, K. Estimates of genetic parameters for mature weight of Australian beef cows and its relationship to early growth and skeletal measures. Livest. Prod. Sci. 1995, 44, 125–137. [Google Scholar] [CrossRef]
- Johnston, D.J.; Chandler, H.; Graser, H.U. Genetic parameters for cow weight and condition score in Angus, Hereford, and Poll Hereford cattle. Aust. J. Agric. Res. 1996, 47, 1251–1260. [Google Scholar] [CrossRef]
- Northcutt, Southward.L.; Wilson, D.E. Genetic parameter estimates and expected progeny differences for mature size in Angus cattle. J. Anim. Sci. 1993, 71, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.E.; Pitchford, W.S. Pick strategies for beef moo-cow size and status. Proc. Assoc. Advmt. Anim. Brood. Genet. 2021, accepted. [Google Scholar]
- Splan, R.1000.; Cundiff, L.V.; Dikeman, Grand.E.; Van Vleck, L.D. Estimates of parameters between direct and maternal genetic effects for weaning weight and direct genetic effects for carcass traits in crossbred cattle. J. Anim. Sci. 2002, 80, 3107–3111. [Google Scholar] [CrossRef]
- Meyer, K.; Carrick, M.J.; Donnelly, B.J.P. Genetic parameters for growth traits of Australian beef cattle from a multibreed selection experiment. J. Anim. Sci. 1993, 71, 2614–2622. [Google Scholar] [CrossRef]
- Kaps, Yard.; Herring, West.O.; Lamberson, W.R. Genetic and environmental parameters for traits derived from the Brody growth curve and their relationships with weaning weight in Angus cattle. J. Anim. Sci. 2000, 78, 1436–1442. [Google Scholar] [CrossRef]
- Cortés-Lacruz, 10.; Casasús, I.; Revilla, R.; Sanz, A.; Blanco, M.; Villalba, D. The milk yield of dams and its relation to directly and maternal genetic components of weaning weight in beef cattle. Livest. Sci. 2017, 202, 143–149. [Google Scholar] [CrossRef]
- Koots, Yard.R.; Gibson, J.P.; Wilton, J.W. Analyses of published genetic parameter estimates for beef production traits. 2. Phenotypic and genetic correlations. Anim. Brood. Abstr. 1994, 62, 825–853. [Google Scholar]
- Wolcott, 1000.Fifty.; Johnston, D.J.; Barwick, S.A.; Corbet, N.J.; Williams, P.J. The genetics of moo-cow growth and trunk composition at commencement calving in two tropical beef genotypes. Anim. Prod. Sci. 2014, 54, 37–49. [Google Scholar] [CrossRef]
- Morris, C.A.; Wilton, J.W. Influence of torso size on the biological efficiency of cows: A review. Can. J. Anim. Sci. 1976, 56, 613–647. [Google Scholar] [CrossRef]
Figure 1. Original size of dataset (black bar) and number (northward) of records after animals with missing contemporary group (CG) and/or age of animal or dam information (blue bar) equally well every bit outliers (orange bar) have been removed from the dataset; n of records were divided by ten for traits presented to the right of the divide; HP = pregnancy charge per unit of fifteen-month-old heifers; DtCH = days to conception in xv-month-old heifers; RB = rebreeding functioning in 2-year-old cows; DtC2 = days to formulation in 2-year-old cows; HWT = live weight of 15-month-old heifers; HBCS = torso status score of 15-month-old heifers; HH = hip summit of 15-month-old heifers; WT2 = live weight of 2-year-old cows; BCS2 = body condition score of 2-year-old cows; HH2 = hip height of ii-year-former cows; MWT = mature cow alive weight; BCS = body condition score of mature cows; MHH = hip height of mature cows; PR = pregnancy rate of mature cows; WWT = weaning weight of calves.
Figure one. Original size of dataset (black bar) and number (northward) of records after animals with missing contemporary grouping (CG) and/or historic period of animal or dam information (blue bar) as well equally outliers (orangish bar) take been removed from the dataset; northward of records were divided by ten for traits presented to the right of the divide; HP = pregnancy rate of fifteen-calendar month-quondam heifers; DtCH = days to conception in xv-month-old heifers; RB = rebreeding performance in 2-year-onetime cows; DtC2 = days to conception in 2-year-old cows; HWT = live weight of 15-month-quondam heifers; HBCS = body condition score of 15-month-old heifers; HH = hip elevation of 15-month-old heifers; WT2 = live weight of 2-yr-old cows; BCS2 = body condition score of 2-twelvemonth-erstwhile cows; HH2 = hip height of two-year-old cows; MWT = mature cow alive weight; BCS = torso condition score of mature cows; MHH = hip tiptop of mature cows; PR = pregnancy rate of mature cows; WWT = weaning weight of calves.
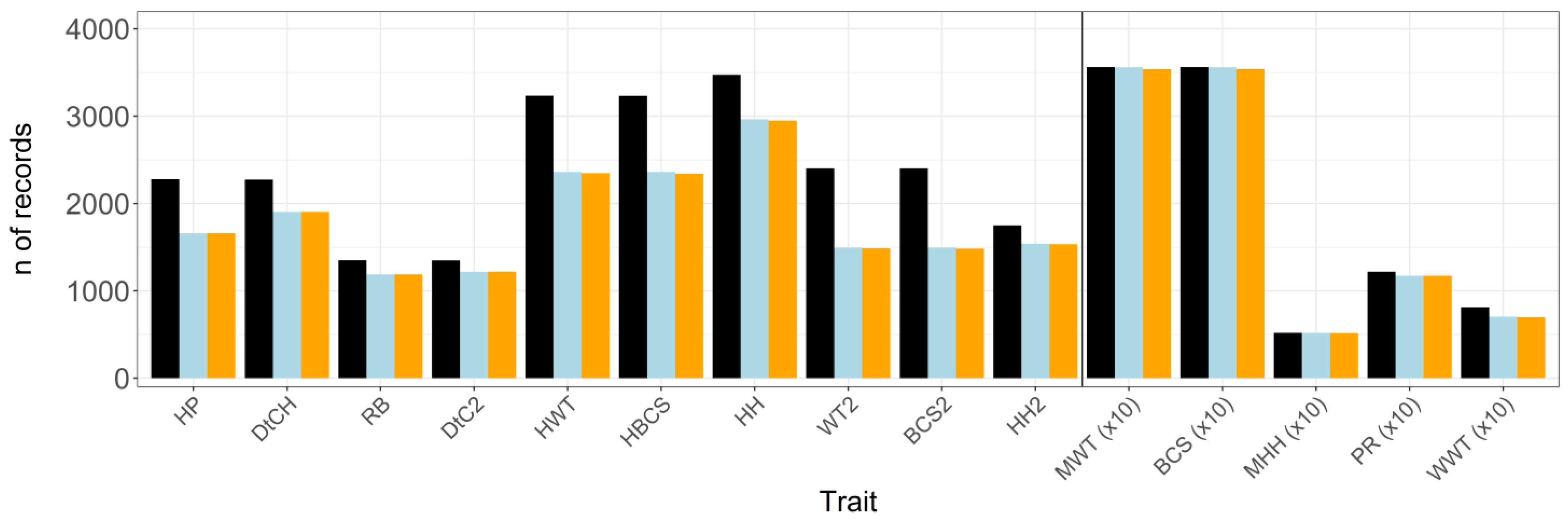
Table 1. Trait abbreviations, units of measurement, number (n) of records, individual animals, sires and dams, range of measurements, means and standard deviations (SD) after adjustments and scaling.
Table i. Trait abbreviations, units of measurement, number (n) of records, individual animals, sires and dams, range of measurements, means and standard deviations (SD) after adjustments and scaling.
| Abb. | Unit | due north of Records | north of Individual Records | n of Dams | due north of Sires | Range | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| Females | Males | |||||||
| Reproduction | ||||||||
| HP | % | 1660 | - | 1660 | 1349 | 232 | 0/one | 88.i 1 |
| DtCH | Days | 1904 | - | 1904 | 1532 | 242 | 0–82 | 24.4 (21.three) |
| RB | % | 1189 | - | 1189 | 1041 | 203 | 0/i | 92.0 one |
| DtC2 | Days | 1220 | - | 1220 | 1072 | 206 | 0–91 | 25.four (21.0) |
| PR | % | 11,730 | - | 4240 | 596 | 148 | 0/i | 93.3 1 |
| Live weight, hip superlative and body status | ||||||||
| HWT | kg | 2347 | - | 2347 | 1822 | 328 | 282–444 | 357.0 (27.1) |
| HBCS | Score | 2340 | - | 2340 | 1822 | 328 | half dozen–9 | 7.9 (0.6) |
| HH | cm | 2948 | - | 2948 | 2185 | 358 | 99–133 | 115.iii (4.7) |
| WT2 | kg | 1488 | - | 1488 | 1265 | 243 | 299–656 | 470.0 (52.four) |
| BCS2 | Score | 1484 | - | 1484 | 1263 | 242 | 4–nine | 7.one (0.8) |
| HH2 | cm | 1535 | - | 1535 | 1295 | 257 | 116–139 | 127.1 (three.9) |
| MWT | kg | 35,375 | - | 4658 | 897 | 195 | 408–728 | 562.4 (47.2) |
| BCS | Score | 35,393 | - | 4660 | 897 | 195 | 3–10 | 6.9 (1.0) |
| MHH | cm | 5172 | - | 3552 | 858 | 186 | 118–143 | 130.3 (iv.0) |
| WWT | kg | 3454 | 3524 | 6978 | 3861 | 381 | 110–338 | 226.five (32.i) |
Tabular array ii. Contemporary group (CG) definitions for each trait and number (n) of CGs.
Table ii. Contemporary grouping (CG) definitions for each trait and number (north) of CGs.
| Traits | CG Definition | n of CGs |
|---|---|---|
| Reproduction | ||
| HP; DtCH | Herd × recording date × birth year × nascency group × weaning group × yearling group × management group at recording | 59; 85 |
| RB; DtC2 | Herd × recording appointment × birth year × direction group at recording | 11; 12 |
| PR | Herd × recording engagement × management grouping | 71 |
| Live weight, hip height and body condition | ||
| HWT; HBCS; HH | Herd × recording date × nascency group × weaning group × yearling group × management grouping at recording | 128–137 |
| WT2; BCS2; HH2 | Herd × recording date × management group at recording | 19–33 |
| MWT; MBCS | Herd × recording date × management group | 247 |
| MHH | Herd × recording date | 18 |
| WWT | Herd × sex × recording engagement × birth management group × weaning management grouping | 189 |
Tabular array iii. Fixed and random effects for each trait included in the variance component analyses.
Table three. Fixed and random furnishings for each trait included in the variance component analyses.
| Fixed Effects | Random Furnishings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age 1 | Age of Dam | Breed of Animal | Heterosis | CG | Directly Genetic | Maternal Genetic | Permanent Environs | Maternal Environment | |
| Reproduction | |||||||||
| HP | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| DtCH | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| RB | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| DtC2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| PR | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Live weight, hip height and body condition | |||||||||
| HWT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HBCS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HH | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| WT2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| BCS2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HH2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| MWT | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| MWTBCS | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| MWTHH | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| BCS | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| MHH | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| WWT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
Tabular array 4. (Co)variance components (
= additive genetic variance,
= maternal genetic variance,
= direct-maternal genetic covariance,
= permanent environmental variance,
= maternal environmental variance,
= rest variance), heritabilities (h2 ± SE) and repeatabilities (t ± SE) from univariate animal models for maternal functioning traits in New Zealand beefiness cattle.
Table 4. (Co)variance components (
= additive genetic variance,
= maternal genetic variance,
= direct-maternal genetic covariance,
= permanent environmental variance,
= maternal environmental variance,
= residual variance), heritabilities (h2 ± SE) and repeatabilities (t ± SE) from univariate animate being models for maternal performance traits in New Zealand beef cattle.
| Model ane | hii | t | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reproduction | |||||||||
| HP | LM | 0.00 | 0.11 | 0.00 | |||||
| THM | 0.xx | iii.29 | 0.06 ± 0.08 | ||||||
| DtCH | LM | two.61 | 430.71 | 0.01 ± 0.05 | |||||
| RB | LM | 0.01 | 0.06 | 0.14 ± 0.09 | |||||
| THM | 0.46 | 3.29 | 0.12 ± 0.eleven | ||||||
| DtC2 | LM | 80.85 | 305.60 | 0.21 ± 0.09 | |||||
| PR | LM | 0.00 | 0.06 | 0.00 | |||||
| THM | 0.00 | three.29 | 0.00 | ||||||
| Live weight, hip summit and torso condition | |||||||||
| HWT | LM | 289.nineteen | 401.08 | 0.42 ± 0.07 | |||||
| HBCS | LM | 0.02 | 0.14 | 0.15 ± 0.05 | |||||
| HH | LM | 5.83 | five.49 | 0.51 ± 0.06 | |||||
| WT2 | LM | 705.23 | 887.29 | 0.44 ± 0.09 | |||||
| BCS2 | LM | 0.09 | 0.26 | 0.25 ± 0.08 | |||||
| HH2 | LM | five.57 | 6.sixteen | 0.47 ± 0.09 | |||||
| MWT | LM | 1157.14 | 790.86 | 481.64 | 0.48 ± 0.04 | 0.80 ± 0.004 | |||
| MWTBCS | LM | 952.07 | 403.78 | 324.36 | 0.57 ± 0.04 | 0.81 ± 0.004 | |||
| MWTHH | LM | 522.81 | 548.30 | 587.32 | 0.32 ± 0.06 | 0.65 ± 0.014 | |||
| BCS | LM | 0.15 | 0.x | 0.34 | 0.26 ± 0.03 | 0.42 ± 0.007 | |||
| MHH | LM | eight.21 | 1.25 | 3.08 | 0.65 ± 0.05 | 0.75 ± 0.010 | |||
| WWTD3 | LM | 84.37 | 122.38 | −53.77 | 187.87 | 235.03 | 0.xiv ± 0.02 | ||
| WWTMiii | LM | 0.20 ± 0.07 | 0.51 ± 0.03 | ||||||
Table 5. Averaged heritabilities (±SEM, diagonal), genetic (±SE, beneath diagonal) and phenotypic (±SE, above diagonal) correlations from bivariate beast models among 15-month-former heifer, two-year-onetime cow and mature cow traits in New Zealand beef cattle.
Table five. Averaged heritabilities (±SEM, diagonal), genetic (±SE, below diagonal) and phenotypic (±SE, above diagonal) correlations from bivariate animal models among 15-month-old heifer, two-year-erstwhile cow and mature cow traits in New Zealand beefiness cattle.
| RB | DtC2 | HWT | HBCS | HH | WT2 | BCS2 | HH2 | MWT | MWTBCS | MWTHH | BCS | MHH | WWT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RB | 0.13 | −0.74 | 0.01 | 0.05 | −0.01 | 0.05 | 0.01 | 0.01 | −0.25 | −0.23 | −0.31 | −0.40 | −0.20 | 0.02 |
| (0.01) | (0.01) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.04) | (0.04) | (0.03) | (0.04) | (0.04) | |
| DtC2 | −0.99 | 0.21 | −0.03 | −0.04 | −0.02 | −0.07 | −0.06 | −0.02 | 0.19 | 0.13 | 0.25 | 0.25 | 0.05 | 0.00 |
| (0.12) | (0.00) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.04) | (0.04) | (0.04) | (0.04) | (0.04) | |
| HWT | 0.xix | −0.23 | 0.45 | 0.38 | 0.61 | 0.73 | 0.26 | 0.53 | 0.54 | 0.62 | 0.32 | 0.17 | 0.48 | 0.79 |
| (0.25) | (0.twenty) | (0.01) | (0.02) | (0.01) | (0.01) | (0.03) | (0.02) | (0.02) | (0.02) | (0.03) | (0.03) | (0.03) | (0.02) | |
| HBCS | 0.49 | −0.32 | 0.26 | 0.15 | 0.12 | 0.23 | 0.25 | 0.05 | 0.09 | 0.06 | 0.12 | 0.12 | −0.03 | 0.26 |
| (0.37) | (0.30) | (0.xvi) | (0.00) | (0.02) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.04) | (0.03) | (0.03) | (0.03) | |
| HH | 0.54 | −0.57 | 0.71 | −0.07 | 0.53 | 0.52 | 0.09 | 0.65 | 0.42 | 0.49 | 0.07 | 0.10 | 0.62 | 0.68 |
| (0.23) | (0.xviii) | (0.06) | (0.17) | (0.01) | (0.02) | (0.03) | (0.02) | (0.03) | (0.02) | (0.04) | (0.03) | (0.02) | (0.02) | |
| WT2 | −0.05 | −0.eleven | 0.84 | 0.00 | 0.66 | 0.52 | 0.57 | 0.54 | 0.74 | 0.79 | 0.47 | 0.35 | 0.59 | 0.57 |
| (0.26) | (0.21) | (0.05) | (0.20) | (0.08) | (0.03) | (0.02) | (0.02) | (0.01) | (0.01) | (0.03) | (0.03) | (0.02) | (0.03) | |
| BCS2 | −0.17 | 0.04 | 0.34 | 0.55 | −0.13 | 0.57 | 0.27 | 0.09 | 0.33 | 0.24 | 0.33 | 0.xl | 0.eighteen | 0.17 |
| (0.32) | (0.27) | (0.xvi) | (0.23) | (0.16) | (0.12) | (0.01) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | (0.03) | |
| HH2 | −0.14 | −0.11 | 0.66 | −0.03 | 0.94 | 0.61 | −0.09 | 0.51 | 0.48 | 0.58 | 0.09 | 0.09 | 0.75 | 0.45 |
| (0.28) | (0.22) | (0.08) | (0.20) | (0.04) | (0.10) | (0.nineteen) | (0.02) | (0.03) | (0.02) | (0.04) | (0.04) | (0.01) | (0.03) | |
| MWT | −0.fifteen | 0.08 | 0.94 | 0.35 | 0.69 | 0.96 | 0.68 | 0.85 | 0.51 | 0.87 | 0.80 | 0.47 | 0.56 | 0.25 1 |
| (0.21) | (0.ten) | (0.05) | (0.12) | (0.05) | (0.03) | (0.09) | (0.05) | (0.01) | (0.00) | (0.01) | (0.01) | (0.01) | (0.02) | |
| MWTBCS | −0.32 | 0.17 | 0.95 | 0.18 | 0.75 | 0.95 | 0.55 | 0.89 | 0.92 | 0.61 | 0.61 | −0.01 | 0.62 | 0.31 ane |
| (0.17) | (0.x) | (0.04) | (0.ten) | (0.04) | (0.02) | (0.09) | (0.04) | (0.01) | (0.02) | (0.01) | (0.01) | (0.01) | (0.02) | |
| MWTHH | −0.xviii | 0.13 | 0.53 | 0.18 | −0.04 | 0.69 | 0.50 | 0.16 | 0.71 | 0.60 | 0.31 | 0.56 | −0.04 | 0.20 |
| (0.26) | (0.19) | (0.11) | (0.17) | (0.09) | (0.11) | (0.15) | (0.12) | (0.04) | (0.06) | (0.00) | (0.01) | (0.02) | (0.03) | |
| BCS | −0.10 | −0.08 | 0.26 | 0.61 | 0.01 | 0.62 | 0.87 | 0.11 | 0.24 | −0.xiv | 0.l | 0.27 | 0.07 | 0.05 |
| (0.nineteen) | (0.xiv) | (0.08) | (0.14) | (0.06) | (0.08) | (0.09) | (0.08) | (0.07) | (0.07) | (0.08) | (0.00) | (0.01) | (0.03) | |
| MHH | −0.27 | −0.04 | 0.61 | −0.16 | 0.88 | 0.64 | −0.03 | 0.97 | 0.77 | 0.lxxx | 0.16 | 0.15 | 0.65 | 0.31 |
| (0.18) | (0.fourteen) | (0.07) | (0.13) | (0.05) | (0.06) | (0.12) | (0.03) | (0.04) | (0.03) | (0.09) | (0.08) | (0.00) | (0.03) | |
| WWTD | −0.08 | −0.05 | 0.87 | 0.52 | 0.56 | 0.70 | 0.51 | 0.69 | 1.00 one | 0.99 1 | 0.64 | 0.89 | 0.53 | 0.16 |
| (0.28) | (0.23) | (0.05) | (0.16) | (0.08) | (0.10) | (0.17) | (0.11) | (0.07) | (0.06) | (0.18) | (0.12) | (0.14) | (0.01) | |
| WWTM | 0.48 | −0.19 | 0.71 | 0.32 | 0.74 | 0.39 | −0.40 | 0.33 | −0.28 i | −0.22 1 | −0.36 | −0.55 | 0.xv | −0.53 2 |
| (0.28) | (0.twenty) | (0.fourteen) | (0.18) | (0.12) | (0.14) | (0.19) | (0.fourteen) | (0.09) | (0.08) | (0.12) | (0.12) | (0.09) | (0.17) |
| Publisher's Annotation: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open up access article distributed nether the terms and conditions of the Artistic Commons Attribution (CC Past) license (https://creativecommons.org/licenses/by/4.0/).
Source: https://www.mdpi.com/2076-2615/11/9/2509/htm
Post a Comment for "Reproductive Traits and Their Heratabilities in Beef Cattle"